Solar energy has been in the works since the late 1800s, when scientists first sought to take the sun’s energy and harness it for our own use. The first solar cell converted only 2% of the sun’s energy into power. Today, that number is about 20% for a typical rooftop solar cell. And in the lab, scientists have been able to capture even more—up to 40% of the sun’s energy.
So, how do solar cells work?
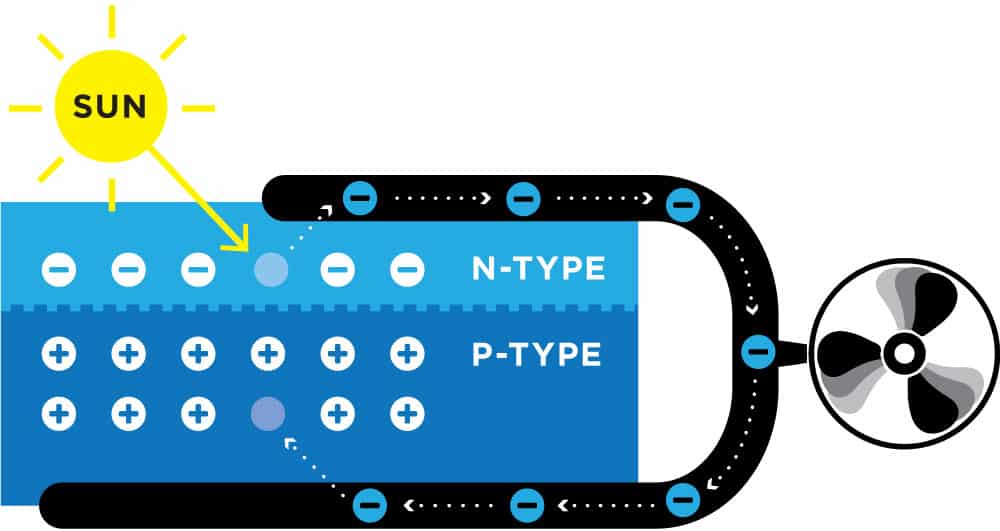
We’re going to need a sandwich. No, not that kind of sandwich. The kind that a solar cell creates with two layers that interact to create a unique filling of energy.
The top layer is doubled up with too many electrons, while the bottom layer has too few. This imbalance makes the electrons want to move around and equalize. But when the sunlight hits them, the electrons get a jolt of energy that causes them to move.
Now free and energized, the electrons in the negative (-) layer can jump through a wire to the underloaded positive (+) layer. This movement between negative and positive is what makes up an electric current.
The circulation of electrons is electricity, and you can use it to turn on lights, cook dinner, watch TV, and even use it as fuel for your car.
The Right Stuff


Another element key to the success of the solar cell is its material. While many different materials and technologies have been tied, including elements like selenium (Se) and germanium (Ge), today silicon (Si) is the basis for most modern solar cells.
Silicon was overlooked for many years because of its unique properties. Unlike other materials, it was not good at “insulating” electricity, and it wasn’t good at “conducting” electricity, either. However, it turned out to be excellent at doing both of these things simultaneously. Silicon is what is called a semi-conductor.
Today, silicon is the primary material used for solar cells but new materials are always being explored.
